Hormonal Contraceptives
Implants
The US Food and Drug Administration (FDA) approved the contraceptive use of levonorgestrel implants (Norplant) in 1990. This method consists of 6 silicone rubber rods, each measuring 34 mm long and 2.4 mm in diameter and each containing 36 mg of levonorgestrel. The implant releases approximately 80 mcg of levonorgestrel per 24 hours during the first year of use, achieving effective serum concentrations of 0.4-0.5 ng/mL within the first 24 hours. The rate of release decreases to an average of 30 mcg/d in the latter years of use. Release of the progestational agent by diffusion provides effective contraception for 5 years. Contraceptive protection begins within 24 hours of insertion if inserted during the first week of the menstrual cycle. The rods are inserted subcutaneously, usually in the woman's upper arm, where they are visible under the skin and can be easily palpated.1,2The mechanism of action is a combination of suppression of the LH surge, suppression of ovulation, development of viscous and scant cervical mucus to deter sperm penetration, and prevention of endometrial growth and development.
Efficacy
The contraceptive efficacy of the method is equivalent to that of surgical sterilization. Overall, pregnancy rates increase from 0.2% in the first year to 1.1% by the fifth year.
Advantages
The longevity of its effectiveness is an advantage. Its effectiveness is not related to its use in regards to coitus. Exogenous estrogen is absent. Prompt return to the previous state of fertility occurs upon removal. No adverse effect on breast milk production occurs.
Disadvantages
A minor surgical procedure is necessary for incision. Difficulty in removal is a disadvantage. Menstrual irregularities are common along with other adverse effects, including headaches, mood changes, hirsutism, galactorrhea, and acne.
Absolute contraindications include active thrombophlebitis or thromboembolic disease, undiagnosed genital bleeding, acute liver disease, benign or malignant liver tumors, known or suspected breast cancer, and a history of idiopathic intracranial hypertension. Relative contraindications include heavy cigarette smoking, a history of ectopic pregnancy, diabetes mellitus, hypercholesterolemia, severe acne, hypertension, and a history of cardiovascular disease, severe vascular or migraine headaches, and severe depression.
Appropriate candidates are women who are postpartum or breastfeeding, women who have difficulty with contraceptive compliance, women in whom pregnancy is contraindicated due to a medical condition, and patients with contraindications to the use of estrogen.
Wyeth-Ayerst Laboratories (Philadelphia, Pa), the maker of the Norplant System, advised health practitioners to inform patients who had the product inserted since October 20, 1999 to use an additional (backup) method of contraception. Laboratory testing showed the product from certain lots may not release enough of the hormone levonorgestrel to deliver effective ongoing contraception. In July 2002, Wyeth Pharmaceuticals then announced that patients who had received the Norplant System and who were using backup contraception may safely stop using backup methods for contraception.
Due to its recall and controversy over use, Wyeth also had announced that it did not plan to resume distribution or marketing of the Norplant System in 2002. The Norplant system had been controversial due to its adverse effects and the company's failure to communicate the risks of adverse effects to the patients. Women alleged that Norplant makers failed to properly and adequately warn them about the severity of adverse effects they may experience, including nausea, headaches, irregular menstrual bleeding, ovarian cysts, weight gain, removal problems, and depression.
Another FDA-approved implant is the 2-rod levonorgestrel system, termed Norplant II or Jadelle. Each rod is 4.4 cm long and contains a cured homogenous mixture of the drug and a polydimethylsiloxane elastomer covered by silicone tubing. Norplant II is approved for 3 years of use but has been shown to be effective for as long as 5 years. After reviewing additional data in 2002, the FDA changed their requirements so as to allow Jadelle to be used for up to 5 years, if it was sold in the United States. The implant has also been approved in Europe for 5 years' use.
Studies have shown Norplant II to have release rates, pregnancy rates, and adverse effect profiles similar to Norplant. At this time, Norplant II is not currently being marketed in the United States. Jadelle is currently available in both developed and developing nations around the world.
Implanon is a single-rod implant that is 4 cm long and 2 mm in diameter. It consists of 68 mg of etonogestrel in an ethylene vinyl acetate copolymer core. Etonogestrel is a biologically active metabolite of desogestrel. Desogestrel is significantly more potent than levonorgestrel; a serum concentration of 0.09 ng/mL can inhibit ovulation in most women. Serum concentrations are adequate for contraception coverage for approximately 3 years. In more than 10 different studies using 4000 women-years of use, no pregnancies have been demonstrated. Effectiveness in women who are overweight has not been defined. Women who weighed more than 130% of their ideal body weight were not studied; because serum concentrations are inversely related to body weight and decrease with time after insertion, etonogestrel implant may be less effective in women who are overweight.
Compared with the Norplant system, Implanon is associated with a higher frequency of amenorrhea and oligomenorrhea, a decrease in the prevalence of frequent and prolonged bleeding, and a decrease in the frequency of adverse effects such as weight gain, headache, and acne. When the rod is removed, the return to fertility is rapid, with the return of ovulation within 3 weeks. Implanon was approved for use in the United Kingdom in 1999 and has recently been approved in the United States. Knowledge of the long-term effects and overall safety data are limited at this point. However, Implanon is not associated with loss of bone mineral density (BMD).
In one study that compared the BMD of patients using Implanon with those using intrauterine devices (IUDs), no decrease was noted in the BMD of either group over a 2-year period.3 Preservation of BMD may, in part, be associated with the observation that women who use Implanon appear to have a greater circulating estradiol concentration than women who use depomedroxyprogesterone acetate (DMPA).
Injectable Depomedroxyprogesterone Acetate
DMPA is a suspension of microcrystals of a synthetic progestin that is injected intramuscularly. Pharmacologically active levels are achieved within 24 hours after injection, and serum concentrations of 1 ng/mL are maintained for 3 months. During the fifth or sixth month after injection, the levels decrease to 0.2 ng/mL, and they become undetectable by 7-9 months after injection.DMPA acts by the inhibition of ovulation with the suppression of follicle-stimulating hormone (FSH) and LH levels and eliminates the LH surge. This results in a relative hypoestrogenic state. Single doses of 150 mg suppress ovulation in most women for as long as 14 weeks. The contraceptive regimen consists of 1 dose every 3 months.
Efficacy
DMPA is an extremely effective contraceptive option. Neither varying weight nor use of concurrent medications has been noted to alter efficacy. Within the first year of use, the failure rate is 0.3%.
Advantages
DMPA does not produce the serious adverse effects of estrogen, such as thromboembolism. Diminished anemia occurs. Dysmenorrhea is decreased. The risks of endometrial and ovarian cancer are decreased. It contains no estrogen, thus making it suitable for women who cannot or will not take estrogen products. It also is safe for breastfeeding mothers.
Disadvantages
Disruption of the menstrual cycle to eventual amenorrhea occurs in 50% of women within the first year. Persistent irregular bleeding can be treated by administering the subsequent dose earlier or by prescribing temporary low-dose estrogen therapy. Because DMPA persists in the body for several months in women who have used it on a long-term basis, it can delay the return to fertility. Approximately 70% of former users desiring pregnancy conceive within 12 months, and 90% of former users conceive within 24 months. Similar to the delay in fertility after discontinuation of DMPA, other adverse effects, such as weight gain, depression, and menstrual irregularities, may continue for as long as 1 year after the last injection.
The FDA issued a "black-box" warning in November 2004, stating that bone loss from using Depo-Provera "may not be completely reversible" even after stopping the drug. The warning urged women not to use Depo-Provera on a long-term basis unless all other methods were inadequate.
Most users of DMPA are teens at a crucial age for the building of bone density; about 10% of American females aged 15-19 years who use birth control use Depo-Provera, compared with 3% of women in the United States overall.
Studies have contradicted the FDA warning. Women who stopped using DMPA experienced an average bone gain of 1.34% at the hip versus a loss of 0.19% for women who never took the drug. Spine density increased 2.86% for women who stopped using the drug, compared with an increase of 1.32% for nonusers. Furthermore, teens regained their bone density faster than older women using Depo-Provera.4
The main limitation, from the patient's point of view, has been the intramuscular (IM) route of injection, which requires an office visit every 12-14 weeks for administration. A subcutaneous version of the drug is now available (depo-subQ provera 104) that delivers a lower dose of medroxyprogesterone acetate (MPA) than does the intramuscular formulation (104 mg vs 150 mg). The subcutaneous route opens the possibility for home self-injections, and the lower dose could decrease suppression of pituitary function and ovarian estradiol production. Further study is needed.
Subcutaneous DMPA, like its intramuscular counterpart, is associated with changes in bone mineral density and also carries a "black box" warning regarding this risk. Studies demonstrate lower decreases in bone mineral density as compared with the intramuscular route and the same reversible effect.
Progestin-Only Oral Contraceptives
Progestin-only oral contraceptives, also known as minipills, are not used widely in the United States. Less than 1% of users of oral contraceptives use them as their sole method of contraception. Candidates for use include women who are breastfeeding and women with contraindications to estrogen use. Two formulations are available, both of which have lower doses of progestin than combined oral contraceptives. One formulation contains 75 mcg of norgestrel. The other has 350 mcg of norethindrone.Prevention of contraception involves a combination of mechanisms similar to, but not as efficacious as, combination oral contraceptives. Mechanisms of action include (1) suppression of ovulation (not uniformly in all cycles); (2) a variable dampening effect on the midcycle peaks of LH and FSH; (3) an increase in cervical mucus viscosity by a reduction in its volume and an alteration of its structure; (4) a reduction in the number and size of endometrial glands, leading to an atrophic endometrium not suitable for ovum implantation; and (5) a reduction in cilia motility in the fallopian tube, thus slowing the rate of ovum transport.
Efficacy
Serum progestin levels peak approximately 2 hours after administration. Within 24 hours, rapid distribution and elimination returns the level to baseline. Greater efficacy is achieved with consistent administration. Failure rates with typical use are estimated to be 7% in the first year of use. However, any variation can increase the failure rate.
Advantages
Due to the lack of estrogen, evidence of serious complications to which estrogen can contribute (ie, thromboembolism) is minimal. Noncontraceptive benefits include decreased dysmenorrhea, decreased menstrual blood loss, and decreased premenstrual syndrome symptoms. Unlike DMPA, fertility is immediately reestablished after the cessation of progestin-only oral contraceptives.
Disadvantages
The most significant disadvantage is the continuous need for compliance with usage. Users need to be counseled on the need for a backup method of contraception if a pill is missed or taken late. A pill is considered late if ingestion occurs 3 hours after the established time of administration. If a pill is missed, it should be taken as soon as possible; the next pill should be taken at the scheduled time. Backup contraception should be used for the next 48 hours. Unscheduled bleeding and spotting are common even with correct use. Other adverse effects include nausea, breast tenderness, headache, and amenorrhea.
Combination Oral Contraceptives
Oral contraceptives have been marketed in the United States since 1962. The dose of sex steroids has declined significantly in the past 40 years. Prior to 1992, the estrogenic component of oral contraceptives consisted of either ethinyl estradiol or mestranol. Today, ethinyl estradiol is used in all preparations containing 35 mcg or less of estrogen in the United States. The progestin component consists of norethindrone, levonorgestrel, norgestrel, norethindrone acetate, ethynodiol diacetate, norgestimate, and desogestrel. The most recent addition to the progestin group is the addition of drospirenone, found in Yasmin birth control pills.The other major development is the reduction in the dosage of ethinyl estradiol to 20 mcg.6 The major impetus for this change is to improve the safety and reduce adverse effects. However, few data exist to indicate whether reduction of the estrogen dose is associated with a decreased risk of serious sequelae. These lower doses are associated with a decrease in the incidence of estrogen-related adverse effects, such as weight gain, breast tenderness, and nausea.
In the United States today, more than 30 oral contraceptive formulations are available. Monophasic oral contraceptives have a constant dose of both estrogen and progestin in each of the hormonally active pills. Phasic combinations can alter either or both hormonal components. Use should be initiated either on the first day of the menses or the first Sunday after menses has begun. Most of the formulations have 21 hormonally active pills followed by 7 placebo pills. This facilitates consistent daily pill intake.
If a woman misses 1 or 2 pills, she should take 1 tablet as soon as she remembers. She then takes 1 tablet twice daily until coverage of the missed pills is achieved. Women who have missed more than 2 consecutive pills should be advised to use a backup method of contraception simultaneous to finishing up the packet of pills until their next menses.
Prevention of ovulation is considered the dominant mechanism of action. Either estrogen or progesterone alone is capable of inhibiting both FSH and LH sufficiently to prevent ovulation. The combination of the 2 steroids creates a synergistic effect that greatly increases their antigonadotropic and ovulation-inhibitory effects. They also alter the consistency of cervical mucus, affect the endometrial lining, and alter tubal transport.
Efficacy
Failure rates are correlated to individual compliance. Rates range from 0.1% with perfect use to 5% with typical use.
Advantages
Oral contraceptives are used as treatment for menstrual irregularity because menses is more regular and predictable. In the prevention of ovulation, oral contraceptives can reduce and sometimes eliminate mittelschmerz. Women with anemia secondary to menorrhagia increase their iron stores. Women can manipulate the cycle to avoid menses during certain events, such as vacations or weekends, by extending the number intake days of hormonally active pills or by skipping the placebo pill week. Oral contraceptives prevent benign conditions, such as benign breast disease, pelvic inflammatory disease (PID), and functional cysts. Functional cysts are reduced by the suppression of stimulation of the ovaries by FSH and LH. Ectopic pregnancies are prevented by the cessation of ovulation.
Oral contraceptives are noted to prevent epithelial ovarian and endometrial carcinoma. Studies have noted an approximate 40% reduced risk of malignant and borderline ovarian epithelial cancer. This protection appears to last for at least 15 years following discontinuation of use and increases with duration of use. This protection has not been studied with low-dose oral contraceptives or in women with genetic ovarian cancer syndromes. Use of oral contraceptives is associated with a 50% reduction of risk of endometrial adenocarcinoma. Protection appears to persist for at least 15 years following discontinuation of use.
Disadvantages
Adverse effects include nausea, breast tenderness, breakthrough bleeding, amenorrhea, and headaches. Oral contraceptives do not provide protection from STDs. Daily administration is necessary, and inconsistent use may increase the failure rate. A few months of delay of normal ovulatory cycles may occur after discontinuation of oral contraceptives. Women who continue to have amenorrhea after a discontinuation period of 6 months require a full evaluation.
Metabolic effects and safety
- Venous thrombosis: The estrogen component of oral contraceptives has the capability of activating the blood clotting mechanism. Use of low-estrogen oral contraceptives is associated with a lower risk of thromboembolism than use of oral contraceptives with higher levels of estrogen. Although use of oral contraceptives is not associated with a detectable hypercoagulable state for most women, users at a greater risk for thromboembolism include women who smoke heavily, women with high or abnormal blood lipids, women with severe diabetes with damage to the arteries, women with consistently elevated blood pressures, and women who are obese.
- Hypertension: Oral contraceptives have a dose-related effect on blood pressure. With the older, high-dose pills, as many as 5% of patients could expect to have blood pressure elevations of 140/90 mm Hg or higher. This elevation is believed to be secondary to an estrogen-induced increase in renin substrate in susceptible individuals. Although today's low-dose pills have minimal blood pressure effects, maintaining a surveillance of blood pressure is advisable.
- Atherogenesis and stroke: Although androgens and a few of the progestins actually may increase low-density lipoproteins and decrease high-density lipoproteins, past use of oral contraceptives does not increase the risk of cardiovascular disease. Limited preliminary data have demonstrated that oral contraceptive use does not lead to coronary atherosclerosis. In rare cases in which myocardial infarcts have been found, the cause has been noted to be of thrombotic rather than of atherosclerotic etiology. In general, a woman's habits are more significant than the use of oral contraceptives in determining her risk for cardiovascular disease. The patient who is sedentary, is overweight, smokes heavily, is hypertensive, is diabetic, or has hypercholesterolemia is clearly at risk.
- Hepatocellular adenoma: These benign liver tumors have been associated with the use of oral contraceptives. Although these tumors are histologically benign, their danger lies in the risk of rupture of the capsule of the liver, leading to extensive bleeding and, possibly, death. With the current low-dose oral contraceptive combination, the risk for liver tumors is much lower.
- Cancer
- The association of oral contraceptive use and breast cancer in young women is controversial. The Collaborative Group on Hormonal Factors in Breast Cancer performed the most comprehensive analysis of breast cancer and oral contraceptive use and reported findings in 1996. This group evaluated original published epidemiological data from more than 20 countries. The results demonstrated that current oral contraceptive users, and those who had used oral contraceptives within the past 1-4 years, had a slightly increased risk of breast cancer. Although these observations support the possibility of a marginally elevated risk, the group noted that the oral contraceptive users had more breast examinations and breast imaging than the nonusers. Thus, although the consensus states that oral contraceptives can lead to breast cancer, the risk is small and the resulting tumors spread less aggressively than usual.
- Current thought is that oral contraceptive use may be a cofactor that can interact with another primary cause to stimulate breast cancer.
- The relationship between oral contraceptive use and cervical cancer is also quite controversial. A weak association may exist between oral contraceptive use and squamous cell cancer of the cervix. Important risk factors include early sexual intercourse and exposure to the human papillomavirus. The overall consensus is that if indeed oral contraceptive use increases the risk of cervical neoplasia, it is a minimal risk. Thus, women who use oral contraceptives should have annual Pap tests.
Contraindications to use include cerebrovascular disease or coronary artery disease; a history of deep vein thrombosis, pulmonary embolism, or congestive heart failure; untreated hypertension; diabetes with vascular complications; estrogen-dependent neoplasia; breast cancer; undiagnosed abnormal vaginal bleeding; known or suspected pregnancy; active liver disease; and age older than 35 years and cigarette smoking.
Lastly, drospirenone has antimineralocorticoid properties. It is contraindicated in patients with kidney or adrenal gland insufficiency or liver problems. Potassium levels should be checked during the first month of use, especially if drospirenone is taken daily with drugs that can increase potassium levels (eg, nonsteroidal anti-inflammatory drugs, ACE inhibitors).
91-Day Combination Oral Contraceptives
Currently on the market, 91-day combination oral contraceptives have touted a reduction in menstrual cycles per year. Seasonale is a 91-day oral contraceptive regimen in which tablets containing the active hormones are taken for 12 weeks (84 d), followed by 1 week (7 d) of placebo tablets. Conventional oral contraceptive use is based on a 28-day regimen (21 d of active tablets followed by 7 d of placebo tablets). Seasonale contains a progestin (levonorgestrel) and an estrogen (ethinyl estradiol), which are active ingredients in already approved oral contraceptives. With the Seasonale dosing regimen, the expected menstrual periods that a woman usually experiences are reduced from once a month to approximately once every 3 months. As with the conventional 28-day regimen, women experience menses while taking placebo tablets.Although Seasonale users have fewer scheduled menstrual cycles, the data from clinical trials show that many women, especially in the first few cycles of use, had more unplanned bleeding and spotting between the expected menstrual periods than women taking a conventional 28-day cycle of oral contraceptive.
To counteract the unplanned bleeding, a newer version of Seasonale (Seasonique) was developed. This new brand completely eliminates the hormone-free interval. Seasonique also has 84 active pills (30 mcg of ethinyl estradiol and 150 mcg of levonorgestrel) but is followed by 7 more active pills (10 mcg ethinyl estradiol) instead of the traditional placebo. Therefore, no hormone-free weeks occur.
The 2 main advantages to replacing the placebo week with a week of low-dose estrogen are a diminished amount of unplanned bleeding and spotting and fewer or no symptoms (eg, cramping, bloating, headaches) for women who are sensitive to the placebo-week hormone fluctuations (in particular, low estrogen). A study of 1000 sexually active adult women (aged 18-40 y) who used Seasonique for one year found that, for cycles 2-4, the median number of bleeding days on a per-patient month was minimal (<1 d).7
The risks of using Seasonale are similar to the risks of other conventional combination oral contraceptives and include an increased risk of blood clots, heart attack, and stroke. The labeling also carries the warning that cigarette smoking increases the risk of serious adverse cardiovascular effects from the use of combination estrogen-containing and progestin-containing contraceptives. The study cited for Seasonique does not report any unexpected adverse events or thromboembolic events; the risk profile is the same as Seasonale.
In 2009, the makers of Seasonique came out with LoSeasonique. LoSeasonique consists of 84 orange tablets containing 0.1 mg levonorgestrel and 0.02 mg ethinyl estradiol and 7 yellow tablets containing 0.01 mg ethinyl estradiol. The risk profile is similar to its sister products Seasonale and Seasonique; however, the risk of unplanned breakthrough bleeding is increased.
In a clinical trial, over a 12-month period, 209 of the 2185 participants (9.6%) discontinued LoSeasonique, at least in part, due to bleeding and/or spotting. This breakthrough bleeding remained consistent over time, averaging 2-3 days of bleeding and/or spotting per each 91-day cycle. The breakthrough bleeding eventually decreased over successive 91-day cycles.
Lybrel is the first FDA-approved oral contraceptive with 365-day combination dosing.8 It contains a low combined daily dose of the hormones levonorgestrel and ethinyl estradiol (90 mcg and 20 mcg, respectively). It provides women with more hormonal exposure on a yearly basis (13 additional weeks of hormone intake per year) than conventional cyclic oral contraceptives that contain the same strength of synthetic estrogens and similar strength of progestins.
The incidence of pill failure that results in pregnancy is approximately 1-2% per year (1-2 pregnancies per 100 women per year of use) if taken every day as directed. The average failure rate is approximately 5% per year (5 pregnancies per 100 women per year of use), including women who do not always take the pill exactly as directed without missing any pills.
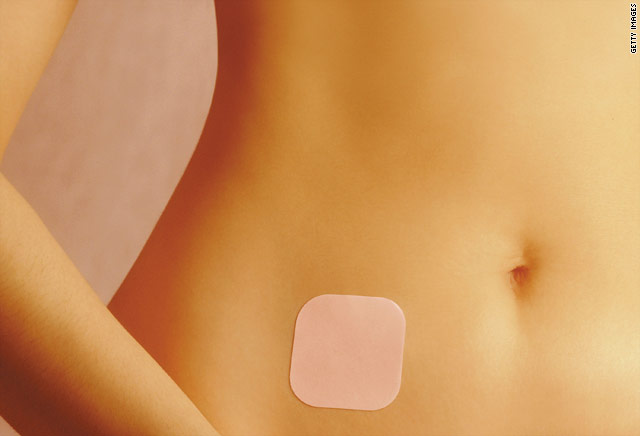
Combination Patch Contraceptive
Available in the United States since 2001, the contraceptive transdermal patch releases estrogen and progesterone directly into the skin (Ortho Evra, Ortho-McNeil Pharmaceutical; Raritan, NJ). Each patch contains a 1-week supply of hormones of both norelgestromin and ethinyl estradiol. It releases a sustained low daily dose of steroids equivalent to the lowest-dose oral contraceptive. In August 2002, the FDA listed a failure rate for the patch of 1 pregnancy per 100 women per year, similar to that of other combination methods. Advantages include greater compliance and decreased adverse effects, such as nausea and vomiting, due to the avoidance of the first-pass effect. However, the patch may cause skin irritation, and, if it is removed unnoticed, such as from showering, this may compromise efficacy. Disadvantages and contraindications are similar to those of combination oral contraceptives. It may be less effective for women who weigh more than 198 pounds.
Ortho Women's Health, a unit of Ortho-McNeil Pharmaceutical Inc, updated the warnings section of the prescribing information for the Ortho Evra patch after new studies revealed that its pharmacokinetic profile differs from the pharmacokinetic profile for combination oral contraceptives. Findings noted a higher steady state concentration and lower peak concentrations in the patch as compared with combination oral contraceptives. Average concentrations at steady state for ethinyl estradiol are approximately 60% higher in women using Ortho Evra and peak concentrations are approximately 25% lower in women using Ortho Evra.
This led to a "black box" warning from the FDA in November, 2005. This announcement warned about higher exposure to estrogen for women using the weekly patch compared with those taking a daily combination oral contraceptive containing 35 mcg of estrogen. The new bolded warning specifically states that women who use Ortho Evra are exposed to about 60% more total estrogen in the blood than if they were taking a typical combination oral contraceptive containing 35 mcg of estrogen. Again, peak blood levels of estrogen are about 25% lower with Ortho Evra than with combination oral contraceptive. While the estrogen level with the patch remains constant for 1 week until the patch is removed, the peak blood levels with a daily combination oral contraceptive rapidly decline to levels that are lower than the Ortho Evra levels. The increased estrogen exposure may increase the risk of side effects, such as a thromboembolic event.
These data contrast with those of another study, conducted by the Boston Collaborative Drug Surveillance Program, which looked at the risk of heart attack, stroke, and venous thromboembolic events in first-time users of the patch. In this study, published online in the journal Contraception, the authors found that "the risk of nonfatal venous thromboembolic events for the contraceptive patch is similar to the risk for oral contraceptives containing 35 mcg of ethinyl estradiol and norgestimate."9 In 2006, the FDA announced that it plans to closely watch the conflicting preliminary data on the risk of thrombosis for women who use Ortho Evra compared with those who take a combination oral contraceptive.
Contraceptive Vaginal Ring
The actual design of vaginal rings as a mode of contraception was first developed in the 1970s.10 The first rings studied were homogenous devices with the steroid mixed uniformly through a polysiloxane matrix. The design was abandoned because of a high initial release of drug with a rapid decrease of drug release thereafter. The vaginal rings can deliver progesterone or progesterone-estrogen combinations. Today, the combination contraceptive vaginal ring is a new form of contraception that was approved by the FDA in October 2001.NuvaRing, a vaginal contraceptive ring developed by Organon (Roseland, NJ), is a nonbiodegradable, flexible, colorless ring made up of a polymer of ethylene vinyl acetate and magnesium stearate. The outer diameter of the ring is 54 mm and the cross-sectional diameter is 4 mm. The ring contains 11.7 mg of etonogestrel and 2.7 mg of ethinyl estradiol. It releases 120 mcg of etonogestrel and 15 mcg of ethinyl estradiol each day. The hormones are released slowly and are absorbed directly by the reproductive organs.
The ring is used in the same schedule as oral contraceptives, with 3 weeks of ring usage (ring is left in place for 3 wk) and 1 week without to produce a withdrawal bleed. The ring can be inserted any time during the first 5 days of the menstrual cycle. The ring should be placed in the vagina even if the woman has not finished bleeding, and she should use a backup contraceptive method for 7 days. A new ring should be inserted each month. If the ring comes out during the first 3 weeks of use, it should be washed with lukewarm water and replaced. If the ring-free interval is more than 3 hours, a backup contraceptive method should be used for 7 days. The ring should never be left in the vagina for more than 4 weeks. If left in for more than 4 weeks, pregnancy should be excluded before inserting a new ring and a backup contraceptive method should be used for 7 days after inserting a new ring.
Gilliam et al compared satisfaction and adherence to the contraceptive vaginal ring (n=136) with daily low-dose oral contraceptive pill (OCP) (n=137) among college and graduate students. Participants completed daily Internet-based, online diaries during 3 cycles and a final online survey at 3 and 6 months.
The authors found that vaginal ring users were more likely to report perfect use during the 3-month trial period than were OCP users. Participants were equally satisfied with their assigned hormonal contraceptive method. At 6 months, less than 30% of participants were still using their assigned method.11
Advantages
NuvaRing is highly effective because it results in complete suppression of ovulation. The steady release of hormone provides exceptional cycle control. The ring is a very effective reversible method of birth control. With typical use, although no studies have been published, the ring is presumed to be more effective than combination oral contraceptives. For example, with typical usage, 8 of 100 pill users become pregnant; with perfect use of the NuvaRing, fewer than 1 of 100 women becomes pregnant.
Because daily intake is not a component of NuvaRing contraception, because it is easily inserted and removed by the woman herself, and because return of fertility is rapid upon discontinuation, NuvaRing is a highly acceptable method for women and their partners. During the clinical trials, women and their partners reported an acceptability rate of 85%. Because the hormones are absorbed directly into the blood through the vaginal mucosa, the hepatic first-pass metabolism of progestin is prevented. The ring delivers the lowest dose of ethinyl estradiol compared with other combined hormonal contraceptives. Unlike combined oral contraceptives, the adverse effects of nausea and vomiting are avoided with ring use. Because NuvaRing is a recently approved product, results of long-term studies will not be available for some time; however researchers assume that the noncontraceptive advantages associated with the NuvaRing will be similar to those known to be associated with the combination oral contraceptives.
Disadvantages
Adverse effects include headaches and vaginal irritation or discharge. The ring may accidentally slip out during intercourse and either the user or the partner may feel the ring during sexual intercourse. Contraindications are similar to those of combined oral contraceptives.
For excellent patient education resources, visit eMedicine's: Men's Health Center and Pregnancy and Reproduction Center.
Also, see eMedicine's patient education articles Birth Control Overview,
Birth Control Barrier Methods,How to Use a Condom,
Vasectomy,
Tubal Sterilization,
Understanding Birth Control Medications (Contraceptives),
Birth Control Hormonal Methods, Birth Control Intrauterine Devices (IUDs)
Emergency Contraception.


[url=http://www.pi7.ru/foto/1503-samye-seksualnye-devushki-18-foto.html ]Интимная проблема [/url]
ReplyDeleteПрочитала,что при половых инфекциях хорошо бы сделать иммунограмму,у меня ген.герпес и часто рецедивы,имеет ли резон мне делать?и что она мне даст?к какому врачу с ней обращаться?еще пропосмотрела,есть иммунограмма скрининговое и расширенная,какая разница в них.Заранее спасибо)
[url=http://www.pi7.ru/otnosheniya/1888-muzhchiny-kotoryh-vybirayut-opytnye-zhenschiny.html ]Лучший , по вашему, крем для лица [/url]
ReplyDeleteВсем привет!Вот решила также начать пользоваться благами прогресса,решила испробовать через интернет покупки обвенчаютсяь,зарегистрировалась на ebay,теперь хочу сыскать асов в этом деле:-)подскажите пожалуйста,какие есть интересные моменты,какие подводные камни?и вообще побольше инфы по этой теме!Заранее спасибо!