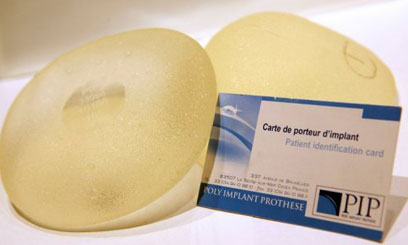
(Reuters) -
Jean-Claude Mas, the Frenchman who sparked a global health scare by
selling substandard breast implants, was arrested on Thursday as
Marseille prosecutors build a case against him for manslaughter.
(Reuters) -
Jean-Claude Mas, the Frenchman who sparked a global health scare by
selling substandard breast implants, was arrested on Thursday as
Marseille prosecutors build a case against him for manslaughter.In the first arrests since the two-year-old scandal made headlines worldwide in December, Mas and a second executive at his now defunct company Poly Implant Prothese (PIP) were seized at their homes in southern France shortly after dawn.
The detention could lead within hours to Mas being placed under formal investigation on suspicion of manslaughter and causing bodily harm. That could in due course lead to criminal charges, which would carry longer sentences than those he now faces in a fraud case expected to be tried around October.
Women who have been campaigning against PIP since French authorities banned its products nearly two years ago welcomed the move as giving them a sense that the law was now in action.
Health authorities in France and elsewhere have stressed that PIP's products carry no proven link to cancer, but surgeons report that they have abnormally high rupture rates. Responses to the problem have varied among different foreign authorities.
Thursday's arrests follow an investigation opened in Marseille, close to PIP's former premises, on December 8 after the death from cancer in 2010 of a woman with PIP implants.Mas and Couty can be held for up to 48 hours while a judge decides whether to open a formal probe and, if so, what bail conditions, if any, to set.A trial date could be years away, given the extent of inquiry required, but the graver manslaughter case could make it harder for Mas to avoid appearing in court later this year on other charges of fraud and deception.
 That latter case targets
half a dozen former PIP executives and could also carry prison terms
for them of several years. It has dragged on as investigators have had
to quiz up to 2,700 women who have filed complaints over PIP implants.
That latter case targets
half a dozen former PIP executives and could also carry prison terms
for them of several years. It has dragged on as investigators have had
to quiz up to 2,700 women who have filed complaints over PIP implants.Mas, who sold some 300,000 implants around the world, has acknowledged that he used unapproved silicone but dismissed fears that it constituted a health risk.
Earlier in January, leaks from a police document showed Mas admitting to lying about the quality of PIP's implants and describing the women filing complaints against him as just seeking money. The comments sparked public anger against him.
PIP closed down in March 2010 after regulators discovered it was using a non-approved, industrial silicone gel, and pulled its implants off the market.
France has called for tighter European Union regulations on medical devices in wake of the PIP affair, saying suppliers of prosthetics should require the same sort of authorization as manufacturers of prescription medicines.
According to estimates by national authorities, over 42,000 women in Britain received the implants, more than 30,000 in France, 9,000 in Australia and 4,000 in Italy. Nearly 25,000 of the implants were sold in Brazil.
Women in 65 countries - mainly in Latin America and elsewhere in Europe - have received implants made by the company, which closed down in March 2010.
Health officials in Germany, the Czech Republic and Venezuela have advised women to have them removed but the medical advice in the UK, where 40,000 are affected, is that there is no need for all the implants to be removed, only those causing problems such as pain or tenderness.
In England, patients fitted with PIP implants by the NHS will have them replaced by the health service, while it will remove implants from private patients if their clinics refuse. The NHS in Wales said it would replace implants only when it was deemed medically necessary.
Women in Northern Ireland who received PIP implants for health reasons will have them replaced, but the NHS will only remove, not replace, those inserted for cosmetic reasons.
Scotland's Health Secretary Nicola Sturgeon said concerned women who had them fitted privately would be offered advice and the option of removal if necessary. There are no records of PIP implants being used by the NHS.